
It corresponds to the charge number of the nucleus. The atomic number uniquely identifies an element chemically. The ordering of the electrons in the ground state of multielectron atoms, starts with the lowest energy state (ground state) and moves progressively from there up the energy scale until each of the atom’s electrons has been assigned a unique set of quantum numbers. Atomic Number of Oxygen: The atomic number or the so-called proton number for any chemical element is the number of protons present in the nucleus of each atom of that chemical element. It is the Pauli exclusion principle that requires the electrons in an atom to occupy different energy levels instead of them all condensing in the ground state. The radioisotopes all have half-lives of less than three minutes. Three are stable, 16 O, 17O, and 18O, of which 16 O is the most abundant (over 99.7). In the periodic table, the elements are listed in order of increasing atomic number Z. in: Molecules, Elements, Isotopes Isotopes of Oxygen Edit Oxygen has seventeen known isotopes with atomic masses ranging from 12.03 u to 28.06 u. The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. Oxygen is a colourless, odourless reactive gas, the chemical element of atomic number 8 and the life-supporting component of the air. The molecular species, O 2, is not especially reactive at normal (ambient) temperatures and pressures. The configuration of these electrons follows from the principles of quantum mechanics. Oxygen is a chemical element with atomic number 8 which means there are 8 protons and 8 electrons in the atomic structure. The intense reactivity of ozone is sometimes explained by suggesting that one of the three oxygen atoms is in an atomic state on reacting, this atom is dissociated from the O 3 molecule, leaving molecular oxygen. Oxygen is a colourless, odourless, tasteless gas essential to living organisms, being taken up by animals, which convert it to carbon dioxide plants, in turn, utilize carbon dioxide as a source of carbon and return the oxygen to the atmosphere. As the only stable isotope of oxygen possessing a nuclear spin (+5/2) and a favorable characteristic of field-independent relaxation in liquid water, 17 O enables NMR studies of oxidative metabolic pathways through compounds containing 17 O (i.e. The chemical properties of the atom are determined by the number of protons, in fact, by number and arrangement of electrons. oxygen (O), nonmetallic chemical element of Group 16 (VIa, or the oxygen group) of the periodic table. Oxygen-17 (17 O) is a low-abundance, natural, stable isotope of oxygen (0.0373 in seawater approximately twice as abundant as deuterium).
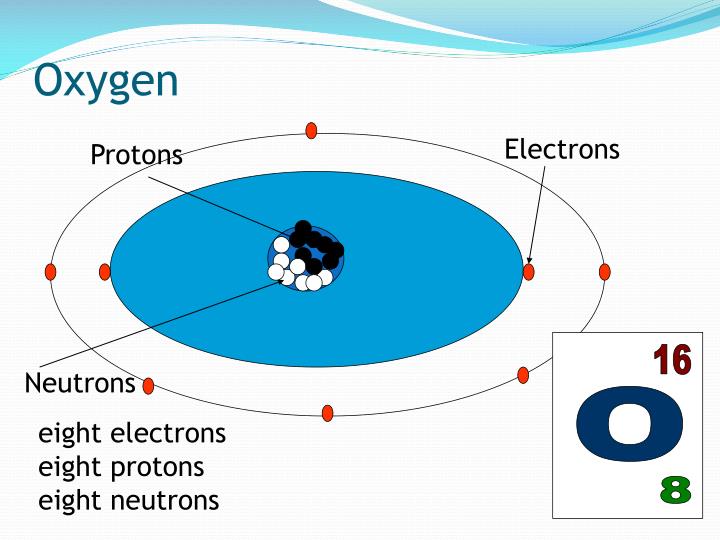
See also: Atomic Number – Does it conserve in a nuclear reaction? Atomic Number and Chemical PropertiesĮvery solid, liquid, gas, and plasma is composed of neutral or ionized atoms. It is the electrons that are responsible for the chemical bavavior of atoms, and which identify the various chemical elements. In a neutral atom there are as many electrons as protons moving about nucleus. The total electrical charge of the nucleus is therefore +Ze, where e (elementary charge) equals to 1,602 x 10 -19 coulombs. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z. The nucleus is composed of protons and neutrons. For example, most noble gases have names ending with -on, while most halogens have names ending with -ine.The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.


Each element has a symbol, which is one or two letters.The periodic table lists the elements in order of increasing atomic number.Each element is identified by the number of protons in its atoms.There are 118 elements on the periodic table.


 0 kommentar(er)
0 kommentar(er)
